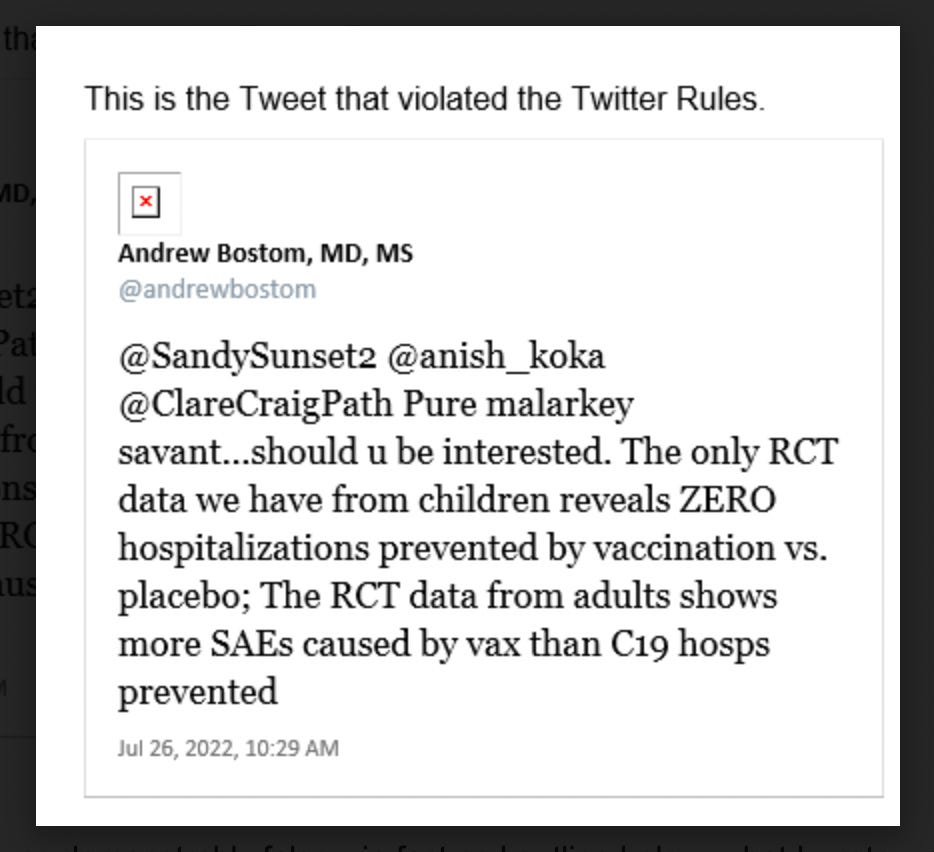
Dr. Andrew Bostom, a long time academic, former NIH funded scientist, and generally a very smart guy was just suspended from Twitter (again) for this tweet.
I’m not a pediatrician and don’t ordinarily read research that leads to approvals for vaccines. I figure some smart people who have the best interest of the health of kids are looking over all of this and making the decisions for all of us. So ordinarily, I’d be skeptical of Dr. Bostom’s claims, but I just had a 90 minute conversation where he comes off as a hyper-intelligent , humble , NIH funded research academic, who seems cautious and nuanced.
Not surprisingly, it turns out that Dr. Bostom’s first sentence is derived from the document the FDA is relying on to approve the vaccine for children. It appears there are three ways the FDA is looking at vaccine efficacy : reduction in symptomatic cases, antibody levels, and reduction in severe disease. Dr. Bostom’s statement relates to vaccine efficacy in the randomized control trials as it relates to the prevention of severe disease related to COVID.
When it comes to reduction in symptomatic cases (figure above), 376 children age 6-23 months were randomized to the Pfizer vaccine (BNT162b2) and 179 participants were randomized to placebo. There was 1 COVID19 case in those receiving the Pfizer vaccine, and 2 cases in the placebo group. This lead to a vaccine efficacy estimate of 75.6%. But the massive paucity of cases here makes this estimate really fragile. This can be seen in the 95% confidence interval that ranges from the vaccine group being 350% more likely to get COVID to 99.6% less likely to get COVID.
When it comes to the specifics of Dr. Bostom’s tweet (severe disease/hospitalization), the relevant section of the FDA document is here:
There was one case of severe COVID reported in the 6-23 month placebo group. There were no cases in the 6-23 month Pfizer group. The initial COVID test in this infant was negative, but a repeat test 9 days after the first negative test was positive. The laboratory testing done at the time also identified a co-infection with rhinovirus/enterovirus. Nine days after this positive test, the child had to be admitted to the hospital for 24 hours after suffering a febrile seizure. The investigator reviewing the case “thought the fever could be attributable to COVID”… though ostensibly it could have been the rhinovirus/enterovirus as well?
For the 2-4 year old age group, there were 7 total severe cases of COVID. Six of the severe cases were in the Pfizer vaccine group, one was in the placebo group. 5 of the 6 severe cases in the Pfizer group were defined as severe because of either an elevated respiratory rate, or an elevated heart rate. In these 5 cases, the investigator reviewing the cases felt the elevated heart rate, and respiratory rate were “not clinically significant” and probably related to “contributing circumstances such as the participant crying during the examination”. That leaves one out of six of the severe covid cases in the vaccine group that was actually hypoxemic and did need to be hospitalized. But even in this case a co-infection with para-influenza was discovered. In the placebo group, the one case of severe COVID was related to hypoxemia and was associated with respiratory symptoms, so it appears pretty straightforward.
For those keeping track, the first part of Dr. Bostom’s tweet appears quite correct as per the FDA documents. In the RCTs available, there does not appear to be evidence that the vaccine prevented hospitalizations.
You can only arrive at the conclusion that the vaccine, in children prevents hospitalization by extrapolating from antibody levels that are achieved in vaccinated children. That’s a much longer topic, but I’ll just say that there’s a reasonable debate to be had in children about what the exact clinical benefit of our current vaccines are given this data. I understand the urge from public health authorities to consider the matter settled to get mass buy-in on a vaccination campaign. The right thing to do is to make their case vigorously in the public square. But banning voices from the public square to make it seem like there is unanimous consensus is wrong and won’t engender trust.
The second part of Dr. Bostom’s tweet is another contention that deserves debate rather than exile. How do rates of serious adverse events actually compare in the randomized control trials to date comparing vaccine and placebo? An interesting pre-print (not peer reviewed) on the topic comes from Doshi and colleagues who re-evaluated serious adverse events in the RCTs to date using a uniform standard definition from March 2020. Using a different definition of serious adverse events, as well as a different time window, the preprint concludes that
“Comparing the excess of serious AESI against the reduction of serious complications of COVID296 19 among the vaccinated is essential for harm-benefit analyses. The results show an excess 297 risk of serious AESIs greater than the reduction in COVID-19 hospitalizations in both Pfizer and 298 Moderna trials.”
Again, the answer from the public health community that disagrees with this statement should be to discuss the paper and its shortcomings. The answer may even be to take the author’s findings seriously enough to run more studies to put the matter to rest. But instead, we are treated to the spectacle of Twitter suspending a longstanding academic and researcher for the crime of discussing science that is being debated in the literature.
It should be fairly clear that there is little chance some twitter intern in the bay area is reading the primary data and coming to a conclusion about real bad faith misinformation versus real scientists having a debate. This smells of a co-ordinated attempt from authorities in government telling tech companies who they need to censor. As Jacinda Arden, dictator of New Zealand puts it: “We will continue to be your single source of truth”

On cue, the Washington Free Beacon broke this exact story a few days ago.
Over the course of at least six months, starting in December 2020, CDC officials regularly communicated with personnel at Twitter, Facebook, and Google over "vaccine misinformation." At various times, CDC officials would flag specific posts by users on social media platforms such as Twitter as "example posts."
In one email to a CDC staffer, a Twitter employee said he is "looking forward to setting up regular chats" with the agency. Other emails show the scheduling of meetings with the CDC over how to best police alleged misinformation about COVID-19 vaccines.
The surgeon general of the United States said as much earlier this Spring in a clear, explicit attempt to pressure platforms to “police misinformation”.
It’s possible the flailing COVID task-force that clearly has no control of a respiratory virus embarked on a campaign to stamp out facebook posts about vaccine microchips because they were faced with an existential crisis. That taxpayer dollars are spent on this endeavor is shameful enough, but based on the suspension of the likes of Dr. Bostom, there is every indication the public authorities are going further to attempt to silence completely valid debates by real scientists on contentious scientific topics.
How completely embarrassing.
Anish Koka is a Cardiologist. Follow him on twitter @anish_koka. Listen to Dr. Bostom’s interview last week on the @Accad_Koka podcast.







Thank you Dr. Koka for your trenchant commentary. I took particular interest in the FDA briefing document to its ad-com in respect of Pfizer's study in 5-11s which led to the EUA. I can't understand why the FDA took seriously the kind of study that Pfizer did. As you know, the study was way too small to show any reduction in deaths--and there were no deaths in either the placebo or vaccine arm of the study. It was also way too small to show any reduction in hospital admissions--and there were no admissions on either arm. The whole exercise rested on antibody titers, even though the FDA and Pfizer agreed there was no established antibody correlate of protection. So the trick became immunobridging. The 5-11s, which I'll call Group A, were bridged to a 16-25s, Group B, but so far as I can tell, no there had been no statistically significant showing of clinical benefit in Group B, but only in a larger group of which Group B was part, that being all the 16 and ups including the middle-aged and the elderly in Pfizer's one big study, which we might call Group C. So follow the bouncing ball: C to B to A. It seems to me Pfizer couldn't even reach its surmise about clinical efficacy except by this double inference. Right? Has any vaccine ever before been authorized on such data? There was no emergency among healthy kids. If Pfizer wanted to show benefit across all kids, it could have done the trial right--enroll a quarter of a million kids. Better, as kids with co-morbidities can be at risk, Pfizer could have done a trial in, say, kids who are immunocompromised, kids who are morbidly obese, etc. In such targeted trials, the populations would have been "enriched," and perhaps then benefit could have clearly demonstrated.
Still unvaxed. Still locked out of Twitter. I suspect the opioid crisis is repeating itself (govt and pharma collusion w/ big tech included this time) and that people have been harmed/killed by the relentless, unhinged, desperate desire to make these shots look safe and effective (they aren’t). Will we learn this time not to blindly trust what the “experts” claim?